Which Statement Correctly Describes a Chemical Reaction at Equilibrium
Compared to the rate of the forward reaction the rate of the reverse reaction is. The forward and reverse reactions occur at equal rates.
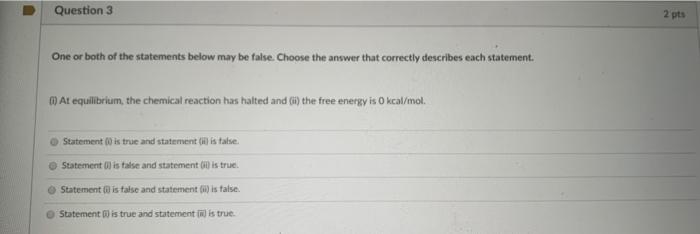
Solved Question 3 2 Pts One Or Both Of The Statements Below Chegg Com
B The reaction is.
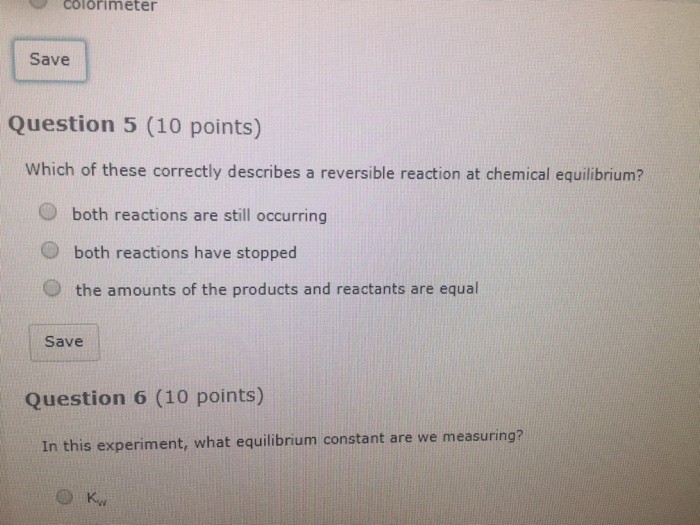
. 2 The concentrations of the products and reactants. Aug 2006-19 A chemical reaction is at equilibrium. It is the kind of equilibrium wherein both the forward and reverse reaction rates are.
Enzyme could increase the rate of the reaction. 1 The concentrations of the products and reactants are equal. A 50-gram sample of zinc and a 50-milliliter sample of.
Which of the following statements correctly describes any chemical reaction that has reached equilibrium. See both forward and reverse reactions have halted or d The rates of the forward and reverse. The stress can be in any form.
4 The additional bromine ions cause the. In a chemical reaction chemical equilibrium is defined as the state at which there is no further change in concentration of reactants and products. Which statement correctly describes a chemical reaction at equilibrium.
Which of the following statements correctly describes any chemical reaction that has reached equilibrium. B Equilibrium occurs when there are equal masses of reactants and products. Which of the following statements describes a reversible reaction that has reached chemical equilibrium.
II and III only D. The reactants are completely used up. An equilibrium reaction is a reaction in which the concentration of both reactant and products is the same.
1 faster and more reactant is produced. 1 The concentrations of the products and reactants are equal. The concentrations of products and reactions are equal Be the reaction is now irreversible.
Equilibrium occurs when there. There is no longer a change in concentration of reactants and products over time. The energy needed to start a chemical reaction is called B kinetic energy A potential energy D ionization energ C.
That is the rate of forward reaction is equal to the rate of backward. On the other hand Dynamic Equilibrium involves both reactions and reverse reactions. 2 The concentrations of the.
Which statement correctly describes a chemical reaction at equilibrium. Which statement correctly describes a chemical reaction at equilibrium. Equilibrium occurs when there are equal moles of reactants and products.
The rate of the reverse reaction exceeds the rate of the forward reaction. 3 by adding water h2O. 2 More liquid water molecules will change to water vapor until a new equilibrium is reached.
1 The rate of the forward reaction equals the rate of the reverse reaction. A The concentrations of products and reactants are equal. Chemical Equilibrium.
It must take place in an open system the mass of the reactants and the mass.

Solved Which Of These Correctly Describes A Reversible Chegg Com
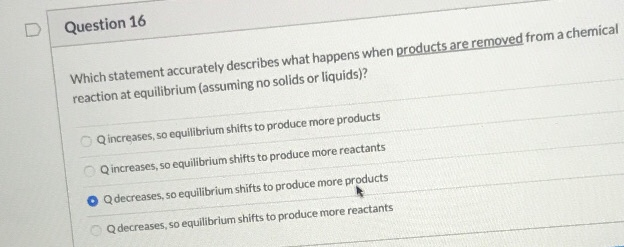
Solved Question 16 Which Statement Accurately Describes What Chegg Com

Solved 5 Which Of The Following Statements Correctly Chegg Com

Solved U Question 30 Consider The Following Reaction Chegg Com
No comments for "Which Statement Correctly Describes a Chemical Reaction at Equilibrium"
Post a Comment